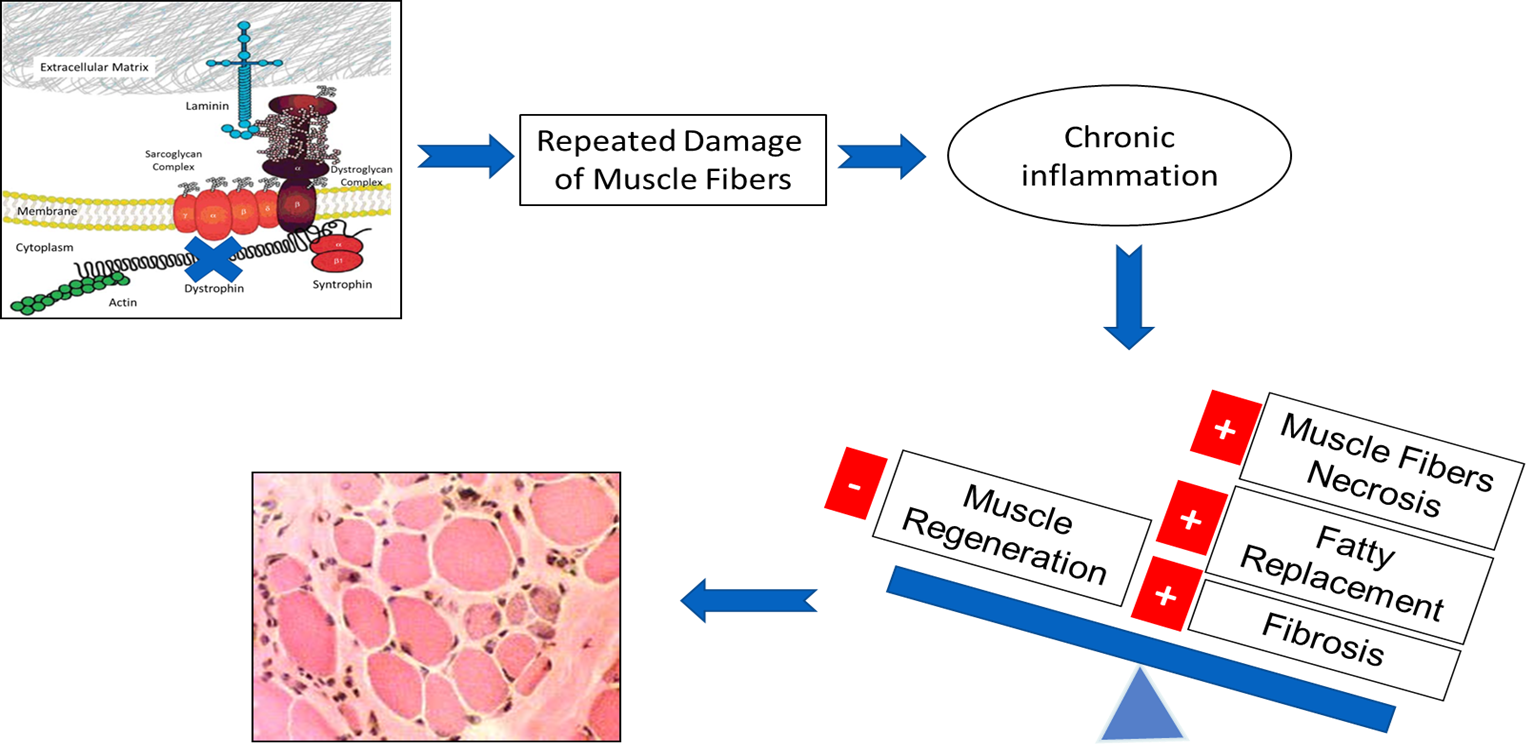
Loss of dystrophin as a result of MD gene mutations disrupts the dystrophin glycoprotein complex, leading to a cascade of events resulting in progressive muscle degeneration with diminished regenerative capacity, satellite cell depletion, and connective tissues replacement. The dystrophin glycoprotein complex confers structural stability to muscular fibers by forming a bridge across the sarcolemma and connecting the basal lamina of the extracellular matrix to the inner cytoskeleton.
In the absence of proper membrane-matrix attachment, mechanical stress from muscle contraction produces defects in the sarcolemma, with influx of calcium through the membranous lesions or through ion channels. Calcium dysregulation activates calcium-dependent proteases to further degrade muscle membrane proteins. Furthermore, loss of nitric oxide results in increased oxidative stress, tissue ischemia, and reparative failure. The progression is clearly visible with replacement of muscle with fibrotic tissue and fat, which leads to severe muscle wasting and weakness.
In dystrophin-deficient muscles, delocalization and downregulation of nNOS leads to hyperactivity of Histone deacetylase (HDAC) which may contribute to the impairment of muscle regeneration and compromise microfiber adaptation to contraction.
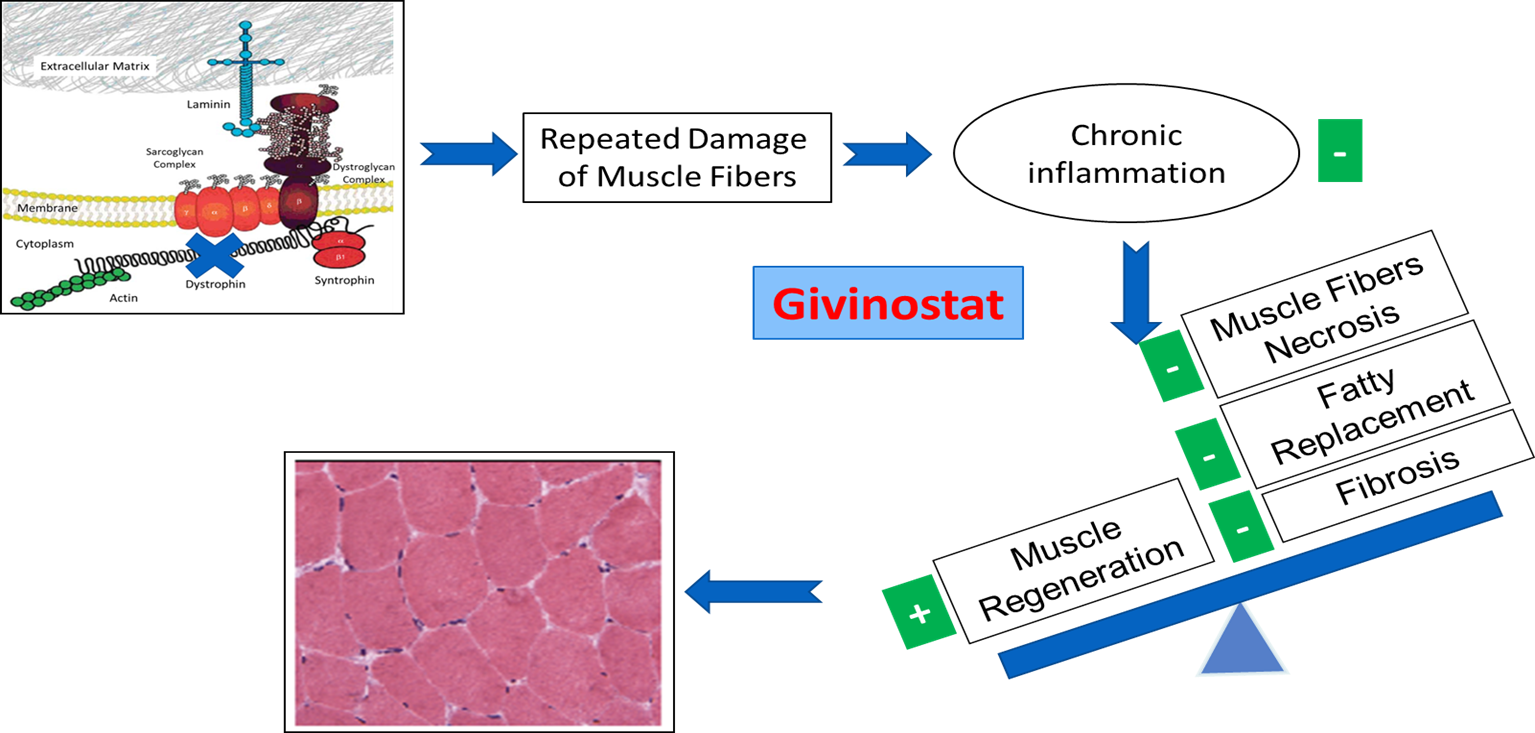
Givinostat, is an HDAC inhibitor (HDACi, a principal candidate, currently being developed for the treatment of DMD and BMD.
Since Givinostat acts on the pathogenetic events downstream of the genetic defects, it is potentially a treatment for the whole DMD and BMD population and to counter the disease pathogenetic events in all muscular districts.
Dystrophic Muscle
Dystrophic Muscle with Givinostat
The FDA has granted Givinostat Orphan Drug, and Fast Track designations for the treatment of DMD and BMD. The European Commission has granted Orphan Medicinal Product designation for Givinostat for the treatment of DMD.
Bettica, P., S. Petrini, V. D'Oria, A. D'Amico, M. Catteruccia, M. Pane, S. Sivo, F. Magri, S. Brajkovic, S. Messina, G. L. Vita, B. Gatti, M. Moggio, P. L. Puri, M. Rocchetti, G. De Nicolao, G. Vita, G. P. Comi, E. Bertini and E. Mercuri (2016). "Histological effects of givinostat in boys with Duchenne muscular dystrophy." Neuromuscul Disord.